Pseudo-Wellens syndrome with Takotsubo cardiomyopathy
A patient was transferred for evaluation for non-ST-segment elevation myocardial infarction.
The patient

A 65-year-old woman with a history of hypertension, mixed hyperlipidemia, type 2 diabetes, and remote smoking history presented to a critical access hospital with intermittent chest pain, dyspnea on exertion, lower-extremity edema, nausea, and vomiting for about one week. Her chest pain was described as an achy, pressure sensation, and her initial troponin level was elevated at 0.397 ng/dL (reference range, 0 to 0.334 ng/dL).
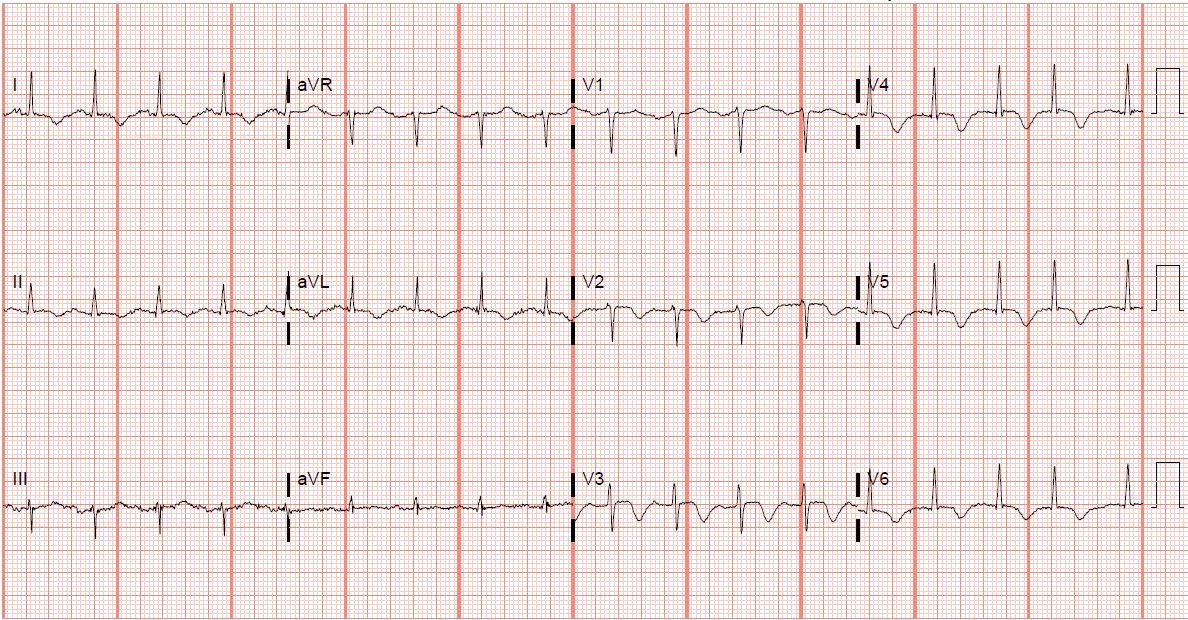
The initial 12-lead electrocardiogram (EKG) showed deep T-wave inversions extending across the precordial leads, lead II, and high-lateral limb leads (Figure 1). The patient was administered aspirin, started on a heparin drip, and transferred to our facility for evaluation for non-ST-segment elevation myocardial infarction.
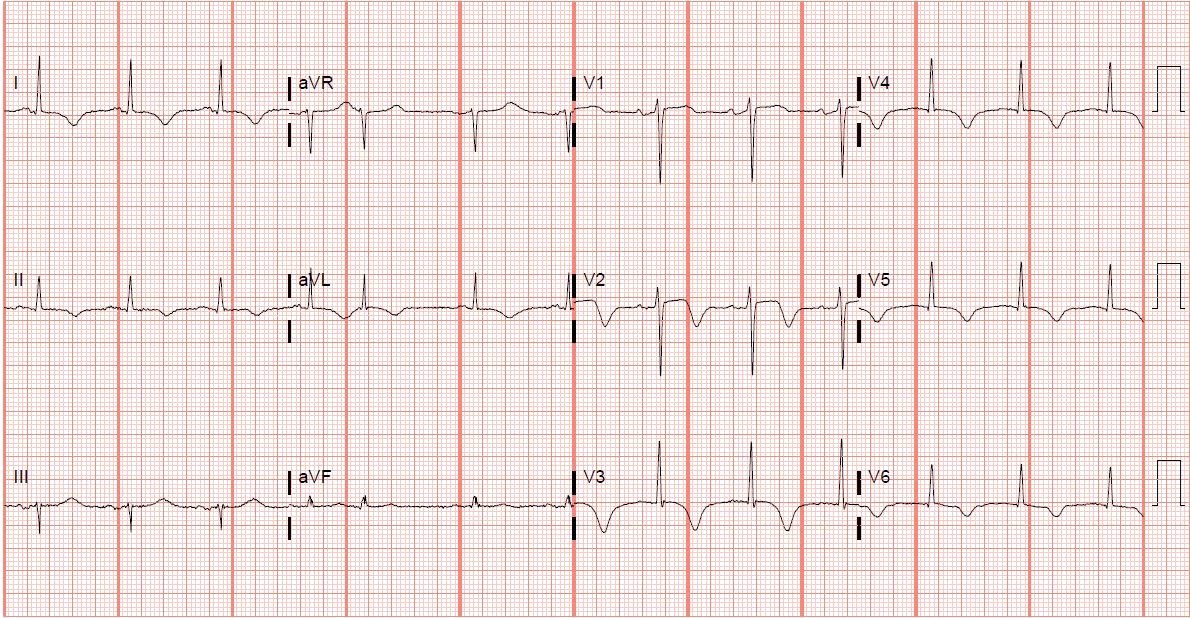
Upon arrival, the patient's troponin level peaked at 0.593 mg/dL. The repeat 12-lead EKG demonstrated dynamic changes in lead V2, now appearing more biphasic, and persistent T-wave inversions across the precordial leads, lead II, and high-lateral leads (Figure 2). Coronary angiography showed a moderately depressed left ventricular ejection fraction, of 35% to 40%, with dyskinesia of the apical anterior wall and apex noted on left ventriculogram. These findings were consistent with Takotsubo cardiomyopathy. Transthoracic echocardiography confirmed apical dyskinesis consistent with Takotsubo cardiomyopathy. The patient was subsequently discharged home with outpatient follow-up planned, including repeat EKGs, goal-directed medical management of her heart failure with reduced ejection fraction, and repeat transthoracic echocardiography.
The diagnosis
The diagnosis is pseudo-Wellens syndrome in a patient with Takotsubo cardiomyopathy. The type A and B pattern were both present in this patient. Type A Wellens pattern is less common and has a biphasic morphology of the T wave with an initial positive deflection. Type B exhibits symmetrical, profoundly inverted T waves. These EKG morphologies may transition between one another, dependent on the transient occlusion and reperfusion of the left anterior descending artery. Pseudo-Wellens syndrome can mimic Wellens syndrome on EKG without the hallmark of proximal left anterior descending artery occlusive coronary disease. Pseudo-Wellens syndrome has been reported with sepsis, pulmonary embolism, marijuana and cocaine use, myocardial bridging, central nervous system injury, acute pancreatitis, cholecystitis, left ventricular hypertrophy, PD-1 monoclonal antibody use, and stress-induced cardiomyopathy. Pseudo-Wellens patterns can be challenging to diagnose, mostly due to the concern for myocardial ischemia and associated need for urgent intervention.
In our case, the pseudo-Wellens pattern was precipitated by Takotsubo cardiomyopathy. The definitive cause of Takotsubo cardiomyopathy is uncertain, but myocardial dysfunction due to catecholaminergic surges after an inciting or stressful event remains the leading theory for pathogenesis. The resulting EKG waveforms appear very similar to what would be expected in Wellens syndrome and can easily be mistaken for occlusive coronary disease, especially if the clinical scenario and laboratory findings suggest the same. Coronary angiography, whether CT or invasive, is recommended in patients with these findings, regardless if the cause is Takotsubo cardiomyopathy or Wellens pattern, to further assess for occlusive coronary disease. However, the management of these two conditions differs considerably, as does the risk for future major adverse cardiac events.
Pearls
- Wellens sign can raise concern for proximal left anterior descending ischemia, especially if classical anginal symptoms are present, but may be due to pseudo-Wellens syndrome.
- Takotsubo cardiomyopathy with apical dyskinesis can mimic a Wellens sign.



