Cases from the University of Massachusetts
Acute drug-induced pancytopenia, liver injury, anaplasmosis, and more.
Case 1: Acute drug-induced pancytopenia
By Yamna Jadoon, MD, ACP Resident/Fellow Member, and Prarthna Bhardwaj, MD, ACP Resident/Fellow Member
The patient

A 72-year-old woman with a history of rheumatoid arthritis (RA) presented to the hospital with generalized weakness, decreased appetite, fever, and shortness of breath for one week. The patient had started oral methotrexate (MTX) for RA six months prior and transitioned to weekly injectable MTX after three months. She had received her most recent dose of intramuscular MTX one week prior to presentation. She had also been hospitalized two weeks prior for acute uncomplicated diverticulitis, treated with levofloxacin and metronidazole. She had a normal complete blood count (CBC) with differential at discharge, and her blood counts had been stable and normal over the previous month.
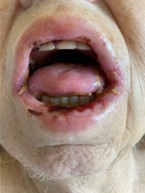
On presentation, she was febrile (temperature, 102.9 oF), tachycardic (heart rate, 123 beats/min), normotensive (blood pressure, 131/73 mm Hg), and hypoxic (oxygen saturation, 93% on 4 L via nasal cannula). Angular cheilitis, mucositis, and stomatitis were noted on exam (Figure 1). The remainder of the exam was unremarkable. Initial labs were significant for pancytopenia with severe neutropenia and a low total leukocyte count of 0.6 k/mm3 (reference range, 4 to 11 k/mm3) and an absolute neutrophil count of 312 cells/µL (reference range, 2,500 to 6,000 cells/µL), normocytic anemia with a hemoglobin level of 8.8 g/dL (reference range, 11.7 to 15.5 g/dL), thrombocytopenia with a platelet count of 21 k/mm3 (reference range, 150 to 460 k/mm3), and a decreased reticulocyte production index of 0.1% (reference range, 1% to 2%). The patient had a normal coagulation profile, normal liver function tests, and no signs of hemolysis. Peripheral smear showed a few atypical lymphocytes but no blasts. An MTX level was drawn on day 2 of admission and was within normal limits.
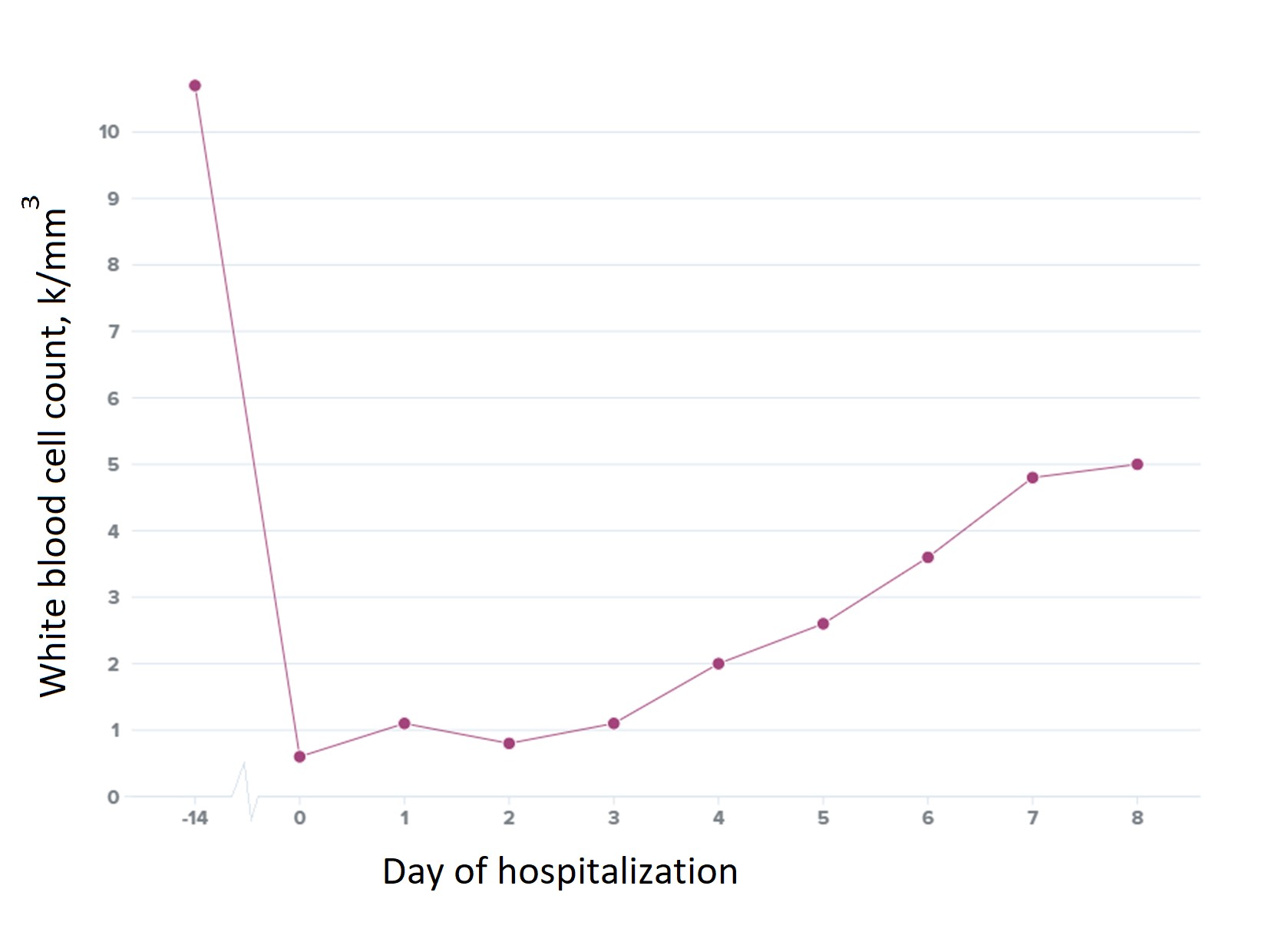
Empiric antibiotic treatment was started with one dose of piperacillin-tazobactam due to concern for intra-abdominal infection, followed by cefepime every eight hours for neutropenic fever of unclear etiology. The patient's symptoms did not improve, and pancytopenia continued to worsen, necessitating platelet transfusion. The patient was empirically treated with high-dose IV leucovorin with dramatic improvement of clinical symptoms and pancytopenia within three days (Figure 2).
The diagnosis
The diagnosis is MTX-induced pancytopenia. The differential diagnosis for acute-onset pancytopenia is broad and includes medication side effects, infections, nutritional deficiencies, hematological malignancies, disseminated intravascular coagulation (DIC), storage diseases, and autoimmune disorders. These cause pancytopenia by one or a combination of the following mechanisms: bone marrow infiltration, bone marrow failure and peripheral destruction, and/or splenic sequestration.
Cytotoxic medications are notorious for causing bone marrow failure/suppression. In an elderly patient presenting with acute-onset pancytopenia, clinical suspicion for MTX-induced toxicity should be high, as such patients are particularly susceptible to MTX-induced myelosuppression and pancytopenia, particularly when transitioning from an oral to a parenteral regimen.
MTX is a folate antagonist and can cause pancytopenia as a result of dose-dependent myelosuppression or as an idiosyncratic drug reaction. MTX-induced pancytopenia is a diagnosis of exclusion. Before it is diagnosed, patients should undergo appropriate workup, including a CBC with differential, peripheral smear, hemolysis labs, DIC labs, and assessment for infections and nutrient deficiencies. Inflammatory marker testing, bone marrow biopsy, and assessment for autoimmune disorders may also be considered. The role of serum MTX level is unclear and normal levels do not rule out the diagnosis, as with this patient. Approximately 1% to 3% of patients treated with MTX experience related pancytopenia. Although no formal guidelines dictate treatment, a well-accepted approach according to existing literature is high-dose folate supplementation and prompt discontinuation of MTX. Concurrent use of bone marrow-stimulating agents such as filgrastim has also been seen to be effective. MTX-induced pancytopenia has a reported mortality rate of 17% to 44%.
Pearls
- Treatment of MTX-induced pancytopenia includes high-dose leucovorin and discontinuation of MTX.
- Patients on MTX should be monitored closely when transitioning from oral to parenteral regimens.
Case 2: Drug-induced liver injury secondary to cefuroxime
By Taroob Latef, MD, ACP Resident/Fellow Member; Muhammad Bilal, MD, ACP Resident/Fellow Member; and Rowha Jawed Latef, MD
The patient
A 54-year-old woman with ulcerative colitis and hypothyroidism presented with a five-day history of constant epigastric abdominal pain associated with nausea. She reported no vomiting or fever. She reported being prescribed cefuroxime, eight days previously, for a headache thought to be secondary to sinusitis. She did not report any use of alcohol, IV drugs, or herbal supplements, or any family history of liver disease. Her only medication was levothyroxine. She couldn't provide information regarding prior cephalosporin use but was sure she had not taken cefuroxime in the past. She was hemodynamically stable with an unremarkable physical examination.
Work-up revealed elevated international normalized ratio of 1.2 (reference range, 0.9 to 1.1), alkaline phosphatase (ALP) level of 214 U/L (reference range, 40 to 129 U/L), lipase level of 853 units/L (reference range, 0 to 160 U/L), total bilirubin level of 4.2 mg/dL (reference range, 0 to 1.2 mg/dL), direct bilirubin level of 2.7 mg/dL (reference range, 0 to 0.3 mg/dL), indirect bilirubin level of 1.5 mg/dL (reference range, 0 to 0.7 mg/dL), aspartate aminotransferase (AST) level of 898 U/L (reference range, 0 to 40 U/L), and alanine aminotransferase (ALT) level of 1,478 U/L (reference range, 0 to 41 U/L). A right upper quadrant ultrasound showed a distended gallbladder without wall thickening and fatty liver with a slightly nodular contour. A CT of the abdomen with contrast revealed no evidence of extrahepatic biliary dilation, pancreatitis, or hepatic steatosis. Urine toxicology was negative. Alpha-1-antitrypsin, smooth-muscle antibody, liver/kidney microsomal antibody, mitochondrial antibody, and ceruloplasmin levels were all within normal limits. Hepatitis testing was negative for hepatitis A, B, C, and E. Iron saturation and ferritin level were elevated at 57% (reference range, 22% to 55%) and 1,722 ng/mL (reference range, 14 to 283 ng/mL), respectively. Cefuroxime was discontinued. A liver biopsy was planned but was deferred as liver tests began to normalize following discontinuation of cefuroxime.
The diagnosis
The diagnosis is drug-induced liver injury (DILI) secondary to cefuroxime with elevated ferritin and lipase levels likely secondary to inflammation and acute liver disease, respectively. Acute viral hepatitis, autoimmune hepatitis, Wilson's disease, and hemochromatosis were ruled out by testing.
DILI accounts for almost 10% of all cases of acute hepatitis. Patients often present with disproportionate elevation in liver function tests, which can manifest as hepatocellular injury (ALT level three or more times the upper limit of normal), cholestatic injury (ALP level two or more times the upper limit of normal), or mixed injury (a combination of the two). Cephalosporins, including cefuroxime, are a rare cause of DILI, reported in only a handful of case reports. Cefuroxime is classified as a less-DILI-concern drug by the FDA. Pathogenesis of DILI secondary to cefuroxime is not completely understood; however, idiosyncratic hepatotoxicity appears to be the common mechanism of most DILI from antibiotics. Other mechanisms include direct drug-induced hepatic injury and immune-mediated reactions. Future use of cephalosporins should be avoided in patients who develop DILI from this class.
A diagnosis of DILI is made after other causes of liver disease are excluded, if drug exposure precedes the onset of liver injury and its discontinuation leads to improvement. The definition also includes reintroduction causing rapid recurrence, although reintroduction may not be advised. A vast variety of medications are associated with DILI, including prescription drugs, over-the-counter medications, and herbal supplements. The most commonly reported are acetaminophen, antibiotics (particularly amoxicillin-clavulanate), and antiepileptics (valproic acid and phenytoin being the highest risk). The NIH has developed a searchable database for these drugs. Treatment involves discontinuation of the culprit drug and serial biochemical measurements to monitor liver function. Glucocorticoids have not been proven to be of benefit but may have some role in patients with hypersensitivity reactions (e.g., pulmonary involvement). If this is suspected, the patient should be evaluated by a hepatologist, as there is no consensus on dosing or regimen. Prognosis depends on the type of DILI, with more favorable outcomes seen with cholestatic injury. Most patients improve after drug withdrawal. All cases of acute liver failure that do not improve should be referred for liver transplant evaluation. Studies have reported poor outcomes, that is, liver transplantation or death, in 9% to 12% of all DILI cases.
Pearls
- The most commonly implicated drug in DILI is acetaminophen, followed by antibiotics (particularly amoxicillin-clavulanate) and antiepileptics.
- Patients often present with vague symptoms, and high suspicion for DILI is warranted, as prompt discontinuation of the offending agent is vital for recovery.
Case 3: Multifocal pneumonia secondary to anaplasmosis
By Aleezay Asghar, MD, ACP Resident/Fellow Member, and Esteban A. DelPilar-Morales, MD
The patient
A 90-year-old woman with stage IIIb chronic kidney disease, chronic obstructive pulmonary disease, and a history of Lyme disease complicated by shoulder arthritis 10 years ago presented with five days of subjective fevers, chills, myalgias, dyspnea on exertion, abdominal pain, decreased appetite, and vomiting. She reported spending most of her days in her garden, with significant exposure to ticks. Vital signs on arrival were significant for a blood pressure of 83/45 mm Hg with a heart rate of 83 beats/min. Physical examination was unremarkable except for abdominal tenderness in the right lower quadrant. Laboratory tests revealed a white blood cell count of 2.9 k/mm3 (reference range, 4.0 to 11.0 k/mm3) with lymphopenia (12.8%; reference range, 15% to 43%) and bandemia (24.0%; reference range, 0% to 5%), and platelet count of 41 k/mm3 (reference range, 150 to 460 k/mm3), as well as an AST level of 67 U/L (reference range, 0 to 32 U/L), ALT level of 44 U/L (reference range, 0 to 33 U/L), and creatinine level of 2.2 mg/dL (patient's baseline, 1.5 to 1.6 mg/dL; reference range, 0.7 to 1.2 mg/dL). A peripheral blood smear was unremarkable. A chest X-ray and a CT of the abdomen and pelvis were also obtained, both unremarkable.
The patient was started on doxycycline for a presumptive diagnosis of anaplasmosis, later confirmed by polymerase chain reaction (PCR) testing. After 48 hours, the patient developed worsening shortness of breath and a nonproductive cough, and her oxygen saturation dropped from 99% to 92% on room air. Bibasilar crackles and rhonchi were noted on examination. Laboratory studies revealed a new leukocytosis of 16.4 k/mm3. A CT of the chest showed bilateral pulmonary parenchymal opacities and pleural effusion concerning for multifocal pneumonia. Blood and sputum cultures were repeated, and Legionella and pneumococcal urinary antigen tests were all unremarkable. The patient continued doxycycline monotherapy for a total of 14 days, with improvement in her symptoms.
The diagnosis
The diagnosis is multifocal pneumonia secondary to anaplasmosis. Anaplasma phagocytophilum is an obligate intracellular bacterium that is primarily transmitted by Ixodes scapularis and pacificus ticks and is hosted by deer and white-footed mice. It is most frequently seen in the upper midwestern and northeastern United States and is especially prevalent during the summer and fall when the transmitting vector is most active. The number of cases of anaplasmosis in the U.S. has been steadily increasing, with over 5,600 reported cases nationwide in 2019. The fatality rate remains less than 1%.
Clinical manifestations of anaplasmosis range from asymptomatic infection to severe illness. Patients typically present with constitutional symptoms and may have leukopenia, thrombocytopenia, and elevated serum aminotransferase levels. Anaplasmosis can be diagnosed with a positive PCR assay, though a negative test does not rule out the disease, especially if obtained after a week or more of symptoms or at least 48 hours of treatment, or if the infection is from another species. The presence of intracytoplasmic inclusions, called morulae, on blood smear is highly specific for anaplasmosis. Serology is also frequently used for diagnosis, though a follow-up sample needs to be obtained two to four weeks after the initial test for confirmation. The CDC recommends beginning treatment if there is clinical suspicion for anaplasmosis; waiting for diagnostic confirmation can increase the risk of morbidity and mortality. Anaplasmosis does not typically present with respiratory symptoms, although respiratory failure has been reported in severe disease. Other potential complications of anaplasmosis include seizures, coma, renal failure, heart failure, pericardial effusion, and demyelinating polyneuropathy, as well as opportunistic infections secondary to leukopenia. Anaplasmosis can in rare cases be associated with hemophagocytic lymphohistiocytosis, a potentially fatal condition. The recommended treatment is seven to 10 days of doxycycline, 100 mg twice daily.
Pearls
- It is important to consider anaplasmosis as a possible diagnosis, especially in endemic regions such as the upper midwestern and northeastern United States, as the disease can have a varied presentation.
- Doxycycline is the first-line treatment for symptomatic anaplasmosis infection and can be initiated with a high index of suspicion, before confirmatory results.
Case 4: Extramedullary hematopoiesis
By Kush Gupta, MD, ACP Resident/Fellow Member, and Prarthna Bhardwaj, MD, ACP Resident/Fellow Member
The patient
A 38-year-old man with a history of beta-thalassemia intermedia, pulmonary hypertension, and dilated cardiomyopathy due to hemochromatosis who had previously undergone defibrillator placement and splenectomy presented to the ED with numbness at and below the level of the umbilicus and difficulty with ambulation. He did not report bowel or bladder incontinence or symptoms to suggest saddle anesthesia. On examination, his vital signs were normal. Physical exam was notable for frontal bossing, thalassemic facies, and pallor. Bilateral lower-extremity strength was preserved, but the sensation to light touch was absent. Laboratory tests were unremarkable.
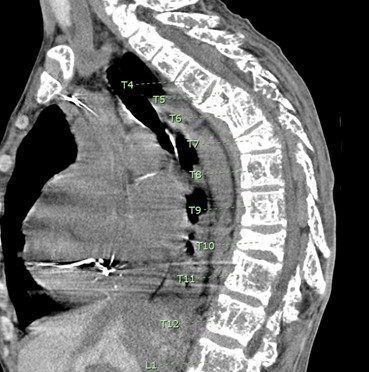
A CT myelogram revealed spinal cord compression between T5 and T10 (Figure 3). An MRI could not be obtained due to the patient's defibrillator.
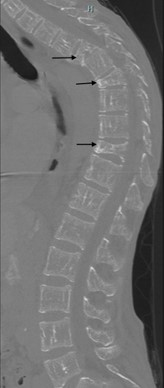
Severe spinal canal stenosis between T5 and T10 due to multiple lobulated masses and moderate compression fracture of T5 was found on a CT scan of the thoracic spine (Figure 4). Given the patient's comorbidities, the extent of the lesions, and the presence of severe osteopenia, it was determined that he was not a good candidate for surgery. Dexamethasone and radiation therapy to T4-T11 levels resulted in improvement of neurologic function. Repeat imaging two days after radiation therapy demonstrated shrinking of the epidural masses with a decrease in the central canal stenosis.
On follow-up imaging one month later, no interval change in the lesions was noted. Hydroxyurea, which the patient had been taking prior to hospitalization, was continued. However, the patient presented to the ED a few months later with neurologic symptoms, including loss of sensation below the abdomen and weakness in bilateral lower extremities. His symptoms were not controlled with steroids, and he had already reached the maximum permissible dose of radiation. He underwent a high-risk posterior T3-T11 decompression surgery with instrumented fusion, with palliative intent. He had a complicated postsurgical hospital course and died in the medical ICU.
The diagnosis
The diagnosis is extramedullary hematopoiesis (EMH). EMH is the production of blood-cell precursors outside the bone marrow and occurs in various hematological diseases, including beta-thalassemia. EMH frequently occurs in the hepatosplenic areas but can also occur paraspinally and may lead to spinal stenosis and spinal compression. It can be seen in up to 20% of cases of nontransfusion-dependent beta-thalassemia, while the incidence is less than 1% in transfusion-dependent thalassemia. Diagnosis is based on signs, symptoms, and imaging. Symptoms include back pain, weakness, loss of sensation, paresthesias, difficulty ambulating, and incontinence. The preferred imaging modality is MRI (with and without contrast). A CT scan is favored when MRI is contraindicated. Needle biopsies are not recommended, given the risks of bleeding in already anemic patients, infection, and paralysis.
There are no guidelines on the management of EMH. Treatment options include steroids, radiation therapy, surgical decompression, and continued management of the condition with hydroxyurea and blood transfusions. Management is aimed at providing temporary symptom relief. Patients should be followed closely for re-emergence of symptoms. Limited data make the mortality rate of this condition uncertain, but the prognosis is poor and clinicians in practice often offer a prognosis of six months to a year.
Pearls
- EMH from hemoglobinopathies can cause spinal-cord compression and symptoms including back pain, weakness, loss of sensation, paresthesias, difficulty ambulating, and incontinence.
- The preferred imaging modality for EMH is MRI, but a CT scan can be used when an MRI is contraindicated.



