Spurious hyperphosphatemia in a multiple myeloma patient
A patient with multiple myeloma had lethargy, disorientation, and several abnormal labs.
The patient

A 75-year-old man with a history of multiple myeloma was brought to the hospital due to lethargy and disorientation. He had been previously treated with daratumumab, pomalidomide, dexamethasone, and denosumab. He had had multiple admissions in the previous year due to hypercalcemia leading to encephalopathy. On physical examination, he was noted to be cathectic with a body mass index of 17.5 kg/m2, lethargic, and disoriented. Blood tests showed hypernatremia with a sodium level of 149 mEq/L (reference range, 135 to 145 mEq/L) and a normal anion gap of 7 mmol/L (reference range, 4 to 12 mmol/L). He had normocytic normochromic anemia with a hemoglobin level of 7.4 g/dL (reference range, 13.8 to 17.2 g/dL; baseline, 7.3 to 8.5 g/dL) and a mean corpuscular volume of 90.8 fl.
Other labs showed thrombocytopenia at 102,000 mcL (reference range, 150,000 to 145,000 mcL) and elevated serum and ionized calcium levels at 17.4 mg/dL (reference range, 8.5 to 10.5 mg/dL) and 2.01 mmol/L (reference range, 1.16 to 1.31 mmol/L), respectively. Serum albumin level was 3.3 g/dL (reference range, 3.4 to 5.4 g/dL); vitamin D3, total bilirubin, and creatine kinase levels were within normal limits. Blood urea nitrogen level was 50 mg/dL (reference range, 6 to 24 mg/dL), and creatinine level was 2.56 mg/dL (reference range, 0.6 to 1.1 mg/dL; baseline, 0.7 to 1.0 mg/dL).
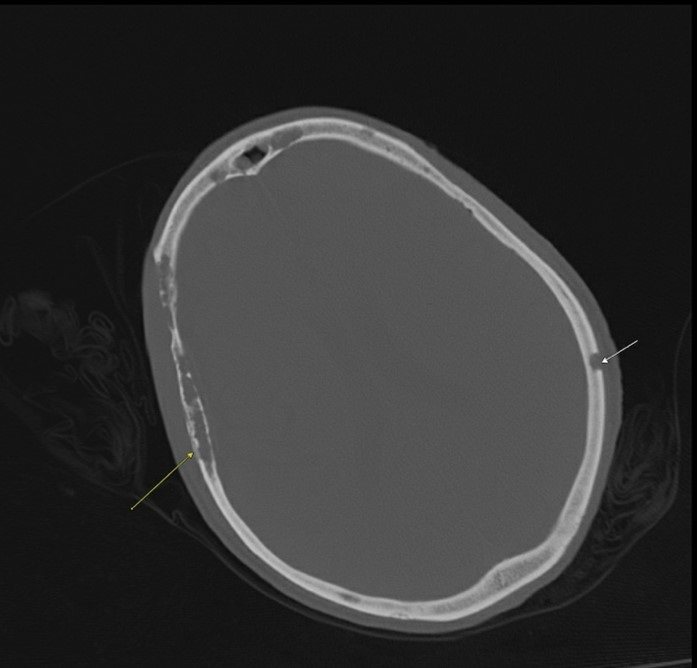
Acute kidney injury was determined to be due to volume depletion in the setting of hypercalcemia. Imaging revealed lytic lesions throughout the calvarium (Figure) and absence of any acute intracranial pathology. The patient was started on IV fluids as the disorientation was thought to be secondary to hypercalcemia. The management plan comprised aggressive fluid resuscitation (IV saline, 200 mL/h); calcitonin, 4 IU/kg twice daily; IV zolendronic acid; and serial monitoring of serum calcium, sodium, and creatinine. Urinalysis showed proteinuria, but there were no indications of remarkable kidney dysfunction.
However, as treatment progressed, serum phosphate levels were noted to increase despite improving kidney function. Serum phosphate levels ranged from 8.3 to 12.6 mg/dL (reference range, 3.0 to 4.5 mg/dL) in the succeeding days. Aluminum hydroxide, 30 cc, was started, but the phosphate levels remained elevated. Serum creatinine levels continued to improve, and the estimated glomerular filtration rate ranged from 54 to 67 mL/min/1.73 m2 (reference range >60 mL/min/1.73 m2). Because of the lack of significant kidney dysfunction, the observed hyperphosphatemia was suspected to be spurious.
We attempted to obtain the true phosphate level through treatment of the serum sample with 20% sulfosalicylic acid or ultrafiltration by contacting multiple hospitals, laboratories, and chemical manufacturers but were not successful. Nonetheless, based on evidence from the literature, we discontinued aluminum hydroxide. After five days of observation, the serum phosphate level spontaneously normalized, and the patient's mental status had been improving throughout the course of hospitalization, so he was discharged.
The diagnosis
The diagnosis is spurious hyperphosphatemia, a false elevation of serum phosphate levels due to analytical interference by monoclonal immunoglobulins in the clinical chemical assays of laboratory automated systems. The mechanism is the interaction of the charges of proteins and pH level of solutions, leading to paraprotein precipitation. Paraproteins bind phosphate, which spuriously increases the total serum phosphate but not the biologically active form of phosphate. Incidence of paraprotein interference can range from 10% to 48.6% of tested samples. In the past 10 years, at least 10 related case reports have been published.
Patients with spurious hyperphosphatemia will not have hyperphosphatemia symptoms (i.e. nausea, vomiting, muscle cramps, seizures, and coma). To avoid unnecessary care, persistent hyperphosphatemia despite treatment should prompt clinicians to search for serum paraproteins prior to performing workup for other causes of an elevated phosphate level.
An easy and inexpensive method to obtain the true phosphate level and the most commonly used strategy in the literature is deproteinization of samples through addition of 20% sulfosalicylic acid to the serum. Other advanced techniques that remove paraproteins by ultrafiltration, ultracentrifugation, dialysis, and immunoabsorption may also be performed but are not always routinely available. In spurious hyperphosphatemia, treatment with phosphate-lowering drugs is unnecessary and will not change the measured phosphate level. Clinicians should be aware of this because aggressive and inappropriate treatments may lead to negative outcomes, including death.
Pearls
- Spurious hyperphosphatemia should be considered when serum phosphate levels increase in a patient with multiple myeloma whose renal function is not remarkably decreased.
- Phosphate-lowering treatments are unnecessary and ineffective in spurious hyperphosphatemia.



